Clinical Data Management
Services
- Clinical Trial Audit & Monitoring
- Clinical Study Design
- Global Regulatory & Clinical Writing
- Clinical Biostatistics
- Clinical Trial Patient Recruitment
- Regulatory Affairs
- Clinical Data Management
- Post-Market Surveillance
- Clinical Technology & Process
- Healthcare Analytics
- Global and Local Literature Search Screening
Clinical Data Management
Clinical data management is proving to be a humungous challenge because of continuous and rigorous monitoring by regulatory authorities in addition to the ever-growing intricacies of clinical trial procedures. This is exactly where Pepgra CRO experts will assist you and take responsibility of your clinical data management project.
Considering these complexities and the basic nature of clinical trials, it essentially warrants the need for a system of clinical management that is state-of-the-art and extensively associated services that go a long way in simplifying the conduct, study design, compliance and management of discrepancies.
The process of gathering, storing and curating copious volumes of clinical data is deemed to be very intrinsic from the perspective of regulatory compliance. Pepgra is a contract research organization engaged in full-fledged and knowledge-based services and offers clinical data management solutions from Phase I through post-marketing trials.
Pepgra offers clinical data management services that are specialized; clinical research organizations can stand to ensure that the quality of data is superior and also offers an assurance as to the integrity of the clinical data. Pepgra assures that the data that is being managed totally and absolutely complies with international standards with a view to ensure consistency within clinical data. In addition, our team also involves the use of data management best practices while adopting specific technologies that are latest to aid the process of clinical data management.
The process of gathering, storing and curating copious volumes of clinical data is deemed to be very intrinsic from the perspective of regulatory compliance. pepgra is a contract research organization that is engaged in full-service knowledge-based diverse activities and offers clinical data management solutions from Phase I through to post-marketing trials.
Pepgra offers clinical data management services that are specialized; clinical research organizations can stand to ensure that the quality of data is superior and also offers an assurance as to the integrity of the clinical data. Pepgra assures that the data that is being managed totally and absolutely complies with international standards with a view to ensure consistency within clinical data. In addition, our team also involves the use of data management best practices while adopting specific technologies that are latest to aid the process of clinical data management
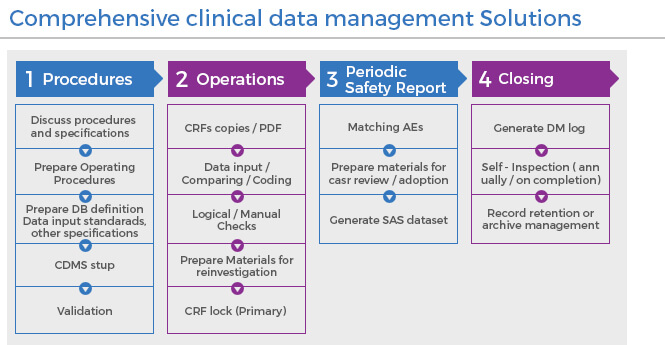
Our team of global clinical data management experts are committed to following a process driven approach thereby developing a truly customized approach for your project requirements. We offer technological expertise in running clinical trials on EDC, CTMS, eTMF platforms and importing data from various devices.
Pepgra has done plethora of work in the area of clinical data management for top pharmaceutical and medical device companies. All our experts comply with international regulatory standards and protocols—in all phases of development.
We deliver clinical study designs that are balanced to meet your business needs with the current scientific understanding and regulatory requirements. We can help you in your clinical data management.
Allow us to help propel your product forward.
Pepgra CRAs did a fabulous job of frequenting the clinical trial sites at different times during the course of study. Apart from their technical know-how, they also had a great affinity with our site team members and finally documented pivotal research findings in the monitoring report which was an eye-opener for us. I would strongly recommend Pepgra as the CRO of choice.
— Barry Stein, VP of a leading medical device manufacturer
We’ll scale
up as your needs grow.
No compromising on integrity and quality. Our processes are well defined and flexible to ramp up as per your requirements.
Partnering with
you till the project end.
We come with you all the way. From design to market support
Pepgra CRO Offerings
"Changing global regulatory system, globalization of clinical trials, increased consumer expectations, infrastructural and culture issues, and various diagnostic requirements should never hamper your research and development programs. With our support..."
Download brochure on our CRO offerings (PDF).



