Clinical trials take up the last half of the 10 – 15 year, 1.5 – 2.0 billion USD, cycle of development just for introducing a new drug within a market. Therefore, any clinical trials services that end in failure tends to translate not just in losses in terms of investment for the clinical trial but also takes into its ambit the costs incurred as preclinical development costs. This renders the loss for every failed clinical trial at 800 million to 1.5 billion USD(Harrer et al., 2019). Keeping these factors in mind, it would be beneficial to explore how Artificial Intelligence or AI as it is popularly known can be effectively utilized to re-mould the key phases of a clinical trial design with a view to augment the rate of success in the trial.

Figure 1 pharma drug development cycle
SourceHarrer et al.,(2019)
Factors causing Failures in Clinical Trials and how AI can help
The main two factors that lead to failure within clinical trials services pertains to the selection of patient cohorts and the mechanisms used for recruiting cohorts. The said two factors are not enough to bring in the most appropriate patients to the trial in a timely manner, along with the absence of a technical infrastructure to match the intricacies of executing a clinical trial(Fogel, 2018). This is particularly true in phases that come later particularly, where there is a lack of an effective and reliable protocol for adherence, clinical endpoint detection systems and patient monitoring, In such scenarios, AI can prove to be extremely beneficial in circumventing such shortfalls of the present clinical trial design. Specifically, deep learning and machine learning would be in a position to automatically seek out meaningful patterns from huge datasets such as speech, text or images. Natural language processing (NLP) as well as Human Machine Interfaces (HMIs) enables a seamless transfer of information amongst humans and computers(Nadkarni et al., 2011).
AI and its Evolution
AI in the domain of medicine has a history which goes back to the early 1970s when proficient systems such as MYCIN had been initially introduced for providing support to diagnostic decisions(Shortliffe, 1984). Nonetheless, early systems of medical AI depended largely on experts from the medical domain for training computers by encrypting clinical knowledge as logic based rules for particular situations or scenarios. Systems of such kind were wrought with the limitation wherein it was intensive to labour and required a lot of time for the construction process. Also, once it was developed there were also considerable challenges with regards to any future updates as such systems were largely rigid(McCauley & Ala, 1992). Highly advanced systems of machine learning which had the ability to train itself to imbibe such rules by recognizing and weighing in pertinent aspects from data like medical images, unstructured texts and electronic health records made an entrance only in the 90s and 2000s. However, the medical domain was slow in terms of adapting such systems mainly owing to the absence of data that was extensively available. This was further aggravated by the fact that such early techniques warranted intense feature engineering efforts that involved severe commitments from experts within the medical domain(Niu et al., 2011). Over the years, the situation improved tremendously especially in recent times, mainly due to two key factors. First and foremost, the domain of AI as such experienced transformational developments, especially in deep learning and machine learning techniques which were facilitated through hardware enhancements and extensive datasets for training(Wang et al., 2019). Secondly, data in the medical domain became largely available and easily accessible in digital formats. Credit goes to innovative developments in technology and also to initiatives on public policy such as the Electronic Records Meaningful Use Programs within the United States of America.
AI in Clinical Trials
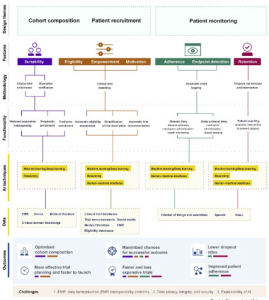
Figure 2 AI for clinical trial design
SourceHarrer et al.,(2019)
The figure above envisions the key techniques through which AI can be introduced within the tenets of clinical design. There are three key themes within the design which comprises of; cohort composition, patient recruitment and patient monitoring. These are founded on the features of the patient in terms of eligibility, suitability, empowerment, enrollment and motivation. This is also inclusive of aspects pertaining to trial such as; adherence control, endpoint detection and retention of patients. An array of methodologies for design has been utilized to execute functionalities of the target. Functionalities such as these are facilitated through distinct combinations from the three key AI technologies – deep / machine learning, human machine interfaces and reasoning wherein every single technology evaluates a particular set of patient and functionality specific sources of data. The pertinent enhancement which is introduced through such type of an execution on the outcome of the study is signified by the length of the horizontal lines within the color bar code beneath the key outcome features. Every study design application that is based on AI directly hinges on the quantum and quality of data it can tap into, and thus is confronted with the similar basic challenges.
References
- Fogel, D.B. (2018). Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemporary Clinical Trials Communications. [Online]. 11. pp. 156–164. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2451865418300693.
- Harrer, S., Shah, P., Antony, B. & Hu, J. (2019). Artificial Intelligence for Clinical Trial Design. Trends in Pharmacological Sciences. [Online]. 40 (8). pp. 577–591. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165614719301300.
- McCauley, N. & Ala, M. (1992). The use of expert systems in the healthcare industry. Information & Management. [Online]. 22 (4). pp. 227–235. Available from: https://linkinghub.elsevier.com/retrieve/pii/037872069290025B.
- Nadkarni, P.M., Ohno-Machado, L. & Chapman, W.W. (2011). Natural language processing: an introduction. Journal of the American Medical Informatics Association. [Online]. 18 (5). pp. 544–551. Available from: https://academic.oup.com/jamia/article-lookup/doi/10.1136/amiajnl-2011-000464.
- Niu, F., Recht, B., Re, C. & Wright, S.J. (2011). HOGWILD!: A Lock-Free Approach to Parallelizing Stochastic Gradient Descent. [Online]. Available from: http://arxiv.org/abs/1106.5730.
- Shortliffe, W.J.C. and E.H. (1984). Readings in Medical Artificial Intelligence: The First Decade. [Online]. Available from: http://people.dbmi.columbia.edu/~ehs7001/Clancey-Shortliffe-1984/Readings Book.htm.
- Wang, F., Casalino, L.P. & Khullar, D. (2019). Deep Learning in Medicine—Promise, Progress, and Challenges. JAMA Internal Medicine. [Online]. 179 (3). pp. 293. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2018.7117.