In brief:
- The clinical study protocol is defined as the procedures by which clinical research is conducted
- A clinical study protocol should provide a clear clinical study design to meet the objective of the clinical trial
- A defined protocol must address the proposed medical question and protect the safety and rights of the clinical trial participant/patients
Introduction
Clinical trials are aimed at evaluating new pharmaceutical drugs, medical devices, and surgical materials, and it is the primary process in identifying the safety and effectiveness of new drugs or devices like a pacemaker for human use. In many cases, clinical trials are used to understand the effectiveness of new drug/devices and damaging side effects. Clinical trials are also conducted in diagnosing diseases early to prevent the severity of the disease conditions. Every new finding of medical drugs and devices goes through four phases of clinical trials to find the appropriate dosage value and the corresponding side effects.
During phase I, the clinical trial experiments will be carried out with a small group of participants to test the safety and side effects to find the accurate dosage value. In phase II, will be testing the effectiveness and obtain preliminary data of curing rate and study the short-term side effects. In phase III, the drug will be tested in a large group to collect more data on safety, effectiveness, side effects related to dosage range with different age groups and disease severity. Upon approval from the FDA at this stage, phase IV will be permitted to carry out with a diverse population for a prolonged period for a detailed study.
Challenges in writing clinical protocol:
The clinical trial protocol is a well-defined procedure of the study, and it must provide a clear & concise design to meet the objective of the study. The protocol for trials evaluating pharmacological drugs, medical devices, and surgical instruments requires complex details that describe ethical, medical and regulatory functions of the clinical trial. In addition, the contributions of medical experts, regulatory experts, statisticians, pharmacokineticists and operational experts are mandatory in protocol development and to address the proposed medical questions.
Writing a well-communicated clinical trial protocol is often challenging, and International Council for Harmonisation Good Clinical Practice provided certain recommendation like what needs to be included in the protocol, structure of clinical study protocol and the standards. Thus, by this time, an experts guidance from Pepgra is preferable as they are experienced in writing many protocols by following clinical protocol structures such as SPIRIT statement and TransCelerate common protocol template and our experts follow ICH E9 standard procedure for a well-constructed clinical trial protocol for all therapeutic area.
General guidelines in writing the clinical protocol:
- Introduction:
The introduction must have detailed literature review covering the existing therapeutic options and test methods.
This section should present the scientific rationale of the clinical trial and identifying the primary purpose includes the aim to achieve and the importance of the clinical study.
- Objective:
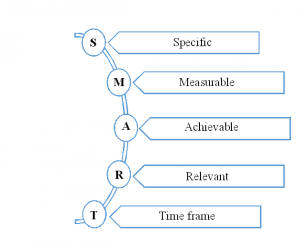
This section should state the objective in the form “SMART”. For a clear delivery of the objective, our experts divide it into a primary, secondary and exploratory category. In which the primary will deliver the aims to answer clinical trial purpose directly, and secondary will explain the associated actions with the rationale. Finally, the exploratory will state the hypothesis-generating objectives that can be analysed with additional studies.
- Population:
Every clinical trial protocol should define the targeted population who will be used for the trial includes the geographical region and a detailed list of inclusion and exclusion criteria which specifies age, sex, BMI and medical conditions.
- Endpoints:
It is defined as an indicator in measuring a biological sample (in this case patient) to assess effectiveness and safety or side effects. In all cases, there should be an endpoint which exactly matches the objective of the study to ensure that the correct variables are measured. In a clinical trial study, variables are parameters which include safety, clinical efficacy, treatment, age, sex, BMI, smoking history, and health outcomes.
- Trial design:
The clinical trial design will indicate the participant’s treatment and the number of required groups for treatment and data collections. If the proposed trial is for the second or third phase, the trial should briefly describe the single group design, which explains the variables comparing the primary phase participants with the exposure to the test treatment. The trial design also needs to ensure the presence of bias minimization. If randomization or blinding are not used in the comparative trial, then bias minimization techniques should be justified in the clinical protocol.
- Control groups:
In the clinical trial protocol, this section explains the control groups and the target population should be clearly identified and justified, and the control group must be aligned with the clinical trial design and objective. Besides, all the experimental tests, including placebo, must be clearly characterized and identified in the clinical trial protocol.
- Statistical considerations:
This section in the protocol should explain the rationale for the calculated sample size with the clinical and statistical assumptions based on the primary endpoint and the statistical analysis must present the complete details of summaries for all endpoints along with the details of the statistical analysis plan. To tackle all the complexity in writing a clinical protocol statistical consideration, we provide statistician support, and when applicable we offer other support, and we have experts like pharmacodynamics/pharmacokinetics experts, quality of life expert, and clinical writing expert.
Conclusion:
In summary, the clinical protocol is the procedure by which the trial is conducted, and the writing requires a wide amount of assessment on all concepts related to the trial. Pepgra has understood the complexity of developing a complete clinical trial protocol with experts. Our team of experts will thus protect the scientific integrity of the trial and the safety of the participants.
Reference:
- Chan A, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158(3):200–7.DOI: 10.7326/0003-4819-158-3-201302050-00583.
- Al-Jundi A, Sakka S. Protocol Writing in Clinical Research. J Clin Diagn Res. 2016;10(11): ZE10–3.DOI: 10.7860/JCDR/2016/21426.8865
- Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol. 2018;44(2):84.DOI: 10.1590/s1806-37562018000000088.
- Jensen JS, Bielefeldt AØ, Hróbjartsson A. Active placebo control groups of pharmacological interventions were rarely used but merited serious consideration: a methodological overview. J Clin Epidemiol.2017;87:35–46.DOI: 10.1016/j.jclinepi.2017.03.001.
- Lim CY, In J. Randomization in clinical studies. Korean J Anesthesiol. 2019;72(3): 221–32.DOI: 10.4097/kja.19049






